
The substitution of cheaper ingredients or the removal of valuable components from common foods is referred to as economic adulteration or economically motivated adulteration. It has been defined as:
[T]he fraudulent, intentional substitution or addition of a substance in a product for the purpose of increasing the apparent value of the product or reducing the cost of its production, i.e., for economic gain. [It] includes dilution of products with increased quantities of an already-present substance (e.g., … watering down of juice) … as well as the addition or substitution of substances in order to mask dilution.
74 Fed. Reg. 15497, 15498 (Apr. 6, 2009).
The concept will be familiar to those who have read (or learned about) Upton Sinclair's 1906 book, "The Jungle," which contributed to passage that year of the federal Pure Food and Drug Act. Addressing in part economic adulteration, the statute declared that food was adulterated, and hence prohibited, if:
First. If any substance has been mixed and packed with it so as to reduce or lower or injuriously affect its quality or strength.
Second. If any substance has been substituted wholly or in part for the article.
Third. If any valuable constituent of the article has been wholly or in part abstracted.
Fourth. If it be mixed, colored powdered, coated, or stained in a manner whereby damage or inferiority is concealed.
35 Stat. 768 § 7 (1906). The concept was carried forward to the 1938 Federal Food, Drug, and Cosmetic Act, and the substance remains largely unchanged to this day. Food is deemed adulterated:
(1) If any valuable constituent has been in whole or in part omitted or abstracted therefrom; or (2) if any substance has been substituted wholly or in part therefor; or (3) if damage or inferiority has been concealed in any manner; or (4) if any substance has been added thereto or mixed or packed therewith so as to increase its bulk or weight, or reduce its quality or strength, or make it appear better or of greater value than it is.
21 U.S.C. § 342(b). Just as the prohibition remains largely unchanged, the problem remains as well.
In December 2022, the U.S. Food and Drug Administration (FDA) released the results of its study of economic adulteration in imported honey, which makes up about 70 percent of the U.S. market. From January 2021 to March 2022, it collected 144 samples of imported honey originating in 32 countries and tested them for the presence of undeclared added sweeteners, such as corn syrup or other plant-derived sugars. About 40 percent of the samples came from India and Vietnam, which are the top two sources of honey imported by the U.S.
Of the 144 samples tested, 14 (10 percent) were found to contain undeclared added sweeteners. As shown in the FDA's chart below, the adulterated products originated from eight different countries:
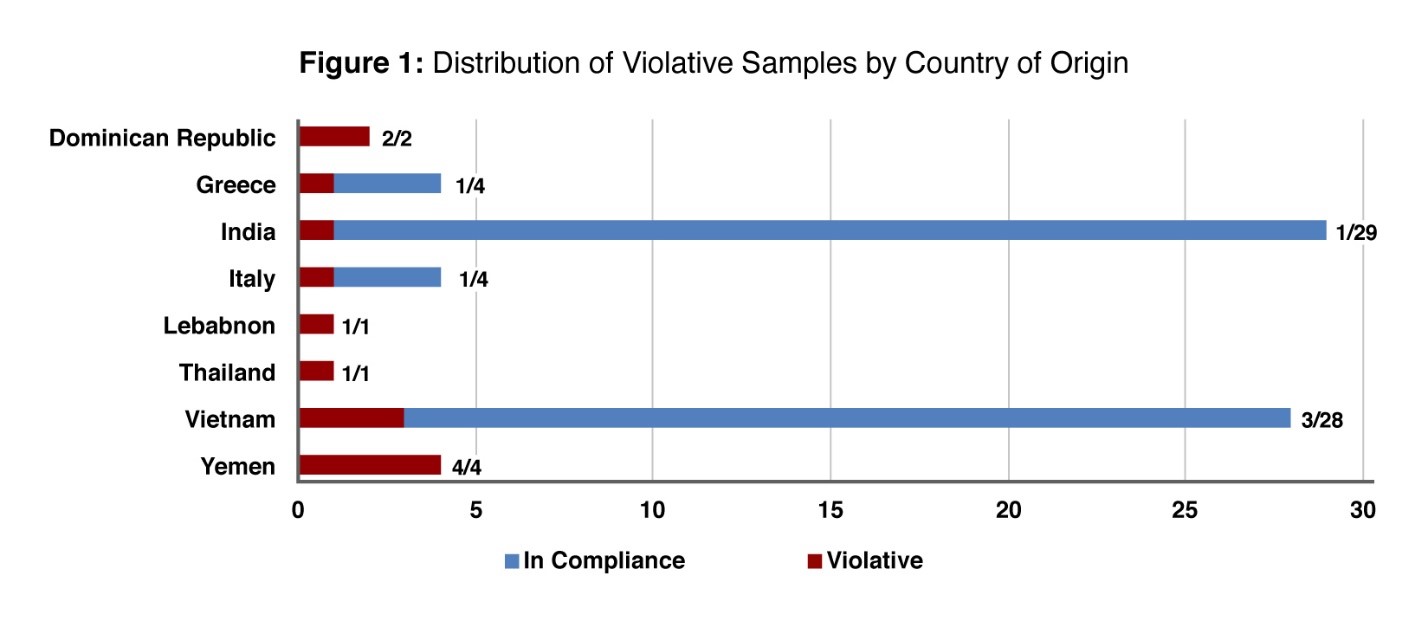
Companies purchasing imported honey in bulk for repackaging for retail or for use in manufacturing should be aware of the potential for economic adulteration. Although in this case the adulteration is not a cause for concern as to safety, the presence of undeclared sweeteners may cause the ingredient listing on labels to be inaccurate if the adulterated product is used with the assumption that it consists only of honey with no added sweeteners.
The good news is that the added sweeteners do not raise a safety issue. The bad news is that they may be fodder for class action cases. Despite their dubious merit, class action cases against food companies commonly allege that product names, slogans, text or graphics on the front of packages suggest the presence or absence of certain ingredients in a manner that misleads consumers, even though review of the products' ingredients list would dispel the claimed misapprehension.
A recent example is a California case alleging that products with the front-of-package statement, "sweetened with monk fruit," are deceptive because they also contain erythritol, a sugar alcohol typically produced from corn. From this, the putative class-representative plaintiff alleges that consumers were willing to pay more for the products because they believed they were sweetened "solely, or at least predominantly," with monk fruit.
Manufacturers have enjoyed a fair degree of success in obtaining early dismissal of these cases. In the case of honey with undeclared sweeteners, there is a risk that the ingredients list itself would be inaccurate, potentially depriving the manufacturer of a solid defense that can support early dismissal.
https://www.hklaw.com/en/insights/publications/2022/12/all-thats-sweet-is-not-honey

